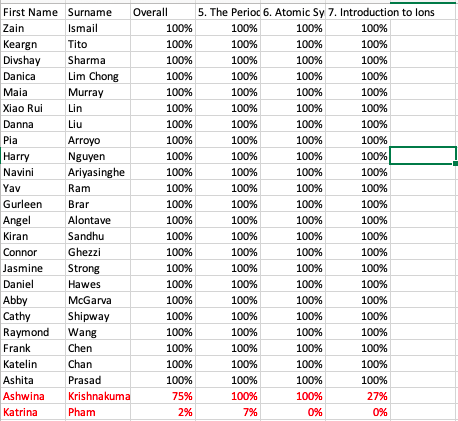
17 February - 23 February
Section outline
-
Week 3: Continuation of last week checking in on our learning before learning about acids and bases.
Success criteria: This week we have been focusing our learning on the Periodic table and reflecting on past knowledge to ensure we have enough knowledge to guide us into learning acids and bases.
Learning objectives: To move forward with acids and bases I need to know the following
- The arrangement of the first 20 elements on the table plus the another 10 key elements.
- How to read the periodic table - atomic number, mass number, protons, neutrons and electrons,
- How to find the groupings - metals, non-metal, alkali metals, alkaline metals, transitional metals, halogens, noble gases, semimetals (metalloids) etc.
- Understand periods (rows - run horizontally) and groups (columns - run vertically).
- Understand how to draw the atomic structure and electron arrangement for the first 20 elements (drawing, table format and how to write a paragraph)
- Atoms are electrically neutral and what this means
Activities:- Developed a table of elements
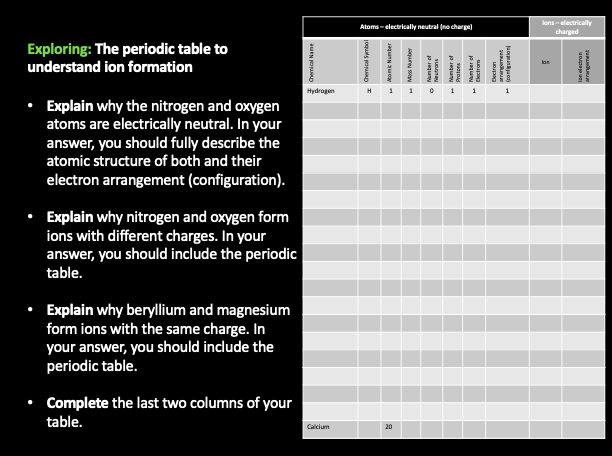
- Developed an understand of how electrically neutral atoms become charged ions
- Explain how atoms become ions using different strategies - tables, paragraphs, atomic structures and electron arrangements.
- Constructed paragraphs using the NCEA writing criteria for science using the three keywords - describe, explain and discuss.
- Reflected on our learning supporting this with Education Perfect activities.
- Watched Video clips

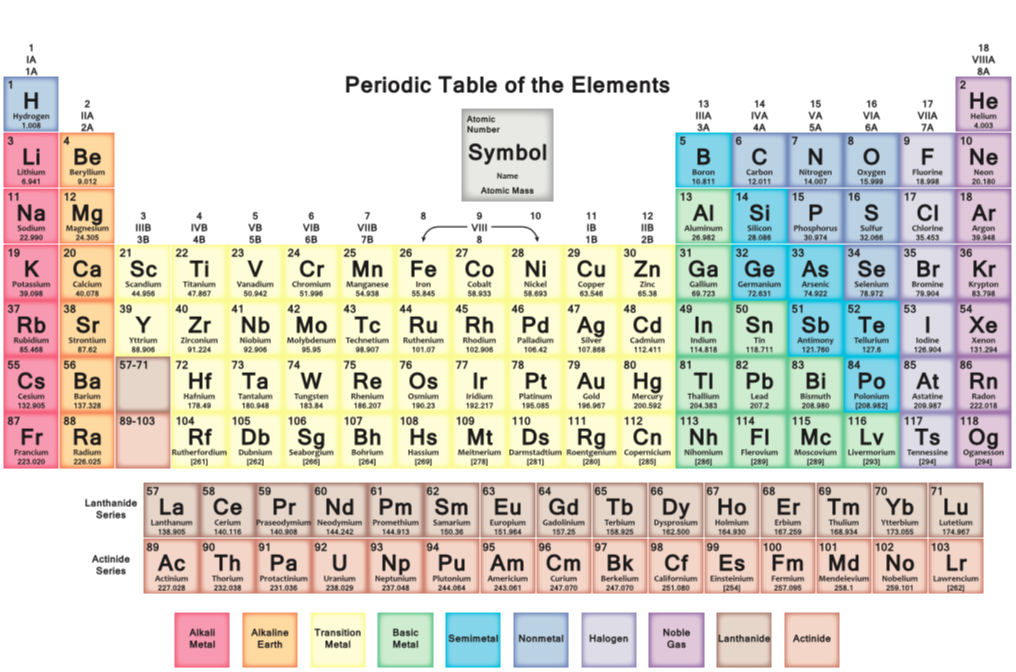


Note:
- From the Periodic table and our learnings about atoms and elements, we have now moved onto understanding the role of ions.
- It is very important that you can complete the success criteria set out below, and understand the keywords.

Homework:
- Incredible week you are an amazing team. Please complete any unfinished work for homework.
- If stuck I am here to help, so please just ask. Education Perfect tasks have been set for you.
- To see me if you are stuck with any tasks. Don't put if off, take ownership of your learning.